Chemical Nomenclature Chart
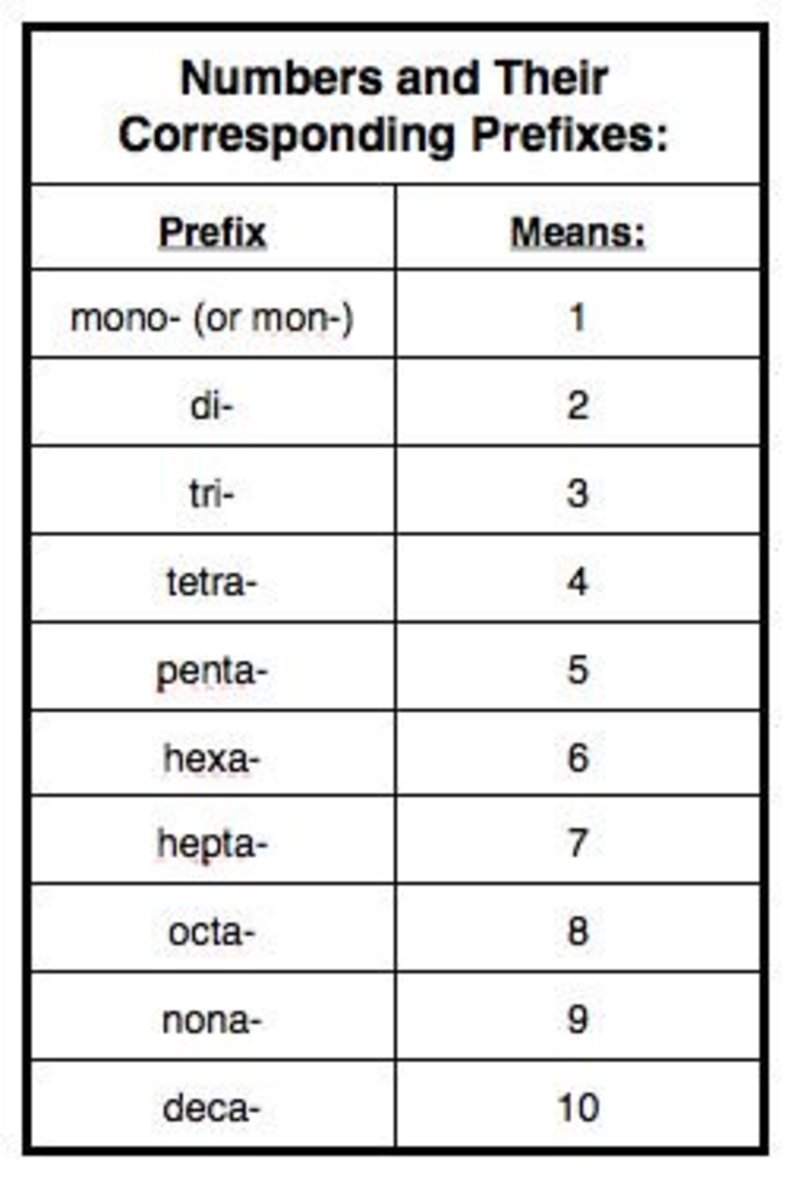
Chemical Nomenclature Chart - These five common classes of compounds are. Web learn the basic rules and principles of nomenclature for organic and inorganic compounds, according to the iupac standards. Chemists use specific rules and conventions to name different compounds. Derive names for common types of inorganic compounds using a systematic approach. Web tutorials and problem sets. In this section, we discuss. This chapter describes an approach that is used to name simple ionic and molecular compounds, such as [latex]\ce{nacl}[/latex], [latex]\ce{caco3}[/latex], and [latex]\ce{n2o4}[/latex]. Long before chemists knew the formulas for chemical compounds, they developed a system of nomenclature that gave each compound a unique name. These compilations are coordinated by the division of chemical nomenclature and. Each chemical name should refer to a single substance. Binary compound (metal/nonmetal) with fixed charge cation. This section is designed to help you review some of those rules and conventions. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). By the end of this section, you will be able to: These compilations are coordinated by the division of chemical nomenclature and. Web nomenclature, a collection of rules for naming things, is important in science and in many other situations. If only one atom of a specific type is present, no subscript is used. Web chemical nomenclature | introduction to chemistry. Chemists use specific rules and conventions to name different compounds. Chemical nomenclature is the term given to the naming of compounds. Chemists use specific rules and conventions to name different compounds. These five common classes of compounds are. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This webpage provides clear examples and explanations for naming different types of molecules and ions, as well as links to other useful resources. Iupac is best. The rules we use in nomenclature depend on the types of bonds the compound has and these are outlined in figure 2.7.1 2.7. In this section, we discuss. The nomenclature used most frequently worldwide is the one created and developed by the international union of pure and applied chemistry (iupac). If only one atom of a specific type is present,. In this section, we discuss. A molecular formula tells us what atoms and how many of each type of atom are present in a molecule. Web in this section we will look at nomenclature of simple chemical compounds. Web iupac nomenclature is used for the naming of chemical compounds, based on their chemical composition and their structure. This chapter describes. Web chemical nomenclature | introduction to chemistry. For naming purposes, a chemical compound is treated as a combination of a parent compound (section 5) and characteristic (functional) groups, one of which is The rules we use in nomenclature depend on the types of bonds the compound has and these are outlined in figure 2.7.1 2.7. The rules we use depends. These five common classes of compounds are. The rules we use depends on the type of compound we are attempting to name. Web chemical nomenclature | introduction to chemistry. Different rules apply to each. The nomenclature used most frequently worldwide is the one created and developed by the international union of pure and applied chemistry (iupac). This chapter describes an approach that is used to name simple ionic and molecular compounds, such as [latex]\ce{nacl}[/latex], [latex]\ce{caco3}[/latex], and [latex]\ce{n2o4}[/latex]. You can choose binary, polyatomic, and variable charge ionic compounds, as well as molecular compounds. Binary compound (metal/nonmetal) with variable charge cation: Web tutorials and problem sets. Web learn the basic rules and principles of nomenclature for organic and. Web the international union of pure and applied chemistry (iupac) is an international federation of organizations that represents chemists in individual countries. This webpage provides clear examples and explanations for naming different types of molecules and ions, as well as links to other useful resources. Web the basics of organic nomenclature, of inorganic nomenclature and polymer nomenclature are summarized in. Web the international union of pure and applied chemistry (iupac) is an international federation of organizations that represents chemists in individual countries. This webpage provides clear examples and explanations for naming different types of molecules and ions, as well as links to other useful resources. Web the following website provides practice with naming chemical compounds and writing chemical formulas. The. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). Chemists use specific rules and conventions to name different compounds. Web in this section we will look at nomenclature of simple chemical compounds. Web the full text of. Web the following website provides practice with naming chemical compounds and writing chemical formulas. Web the primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to: Derive names for common types of inorganic compounds using a systematic approach. Binary compound (metal/nonmetal) with variable charge. Web chemical nomenclature is a set of rules to generate systematic names for chemical compounds. Web learn the basic rules and principles of nomenclature for organic and inorganic compounds, according to the iupac standards. The rules we use depends on the type of compound we are attempting to name. This chapter describes an approach that is used to name simple ionic and molecular compounds, such as [latex]\ce{nacl}[/latex], [latex]\ce{caco3}[/latex], and [latex]\ce{n2o4}[/latex]. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. You can choose binary, polyatomic, and variable charge ionic compounds, as well as molecular compounds. If only one atom of a specific type is present, no subscript is used. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). By the end of this section, you will be able to: This section is designed to help you review some of those rules and conventions. Names that are arbitrary (including the names of the elements, such as sodium and hydrogen) as well as laboratory shorthand names (such as diphos and lithal) are For atoms that have two or more present, a subscript. A molecular formula tells us what atoms and how many of each type of atom are present in a molecule. Chemists use specific rules and conventions to name different compounds. Web specialists in nomenclature recognise two different categories of nomenclature. For naming purposes, a chemical compound is treated as a combination of a parent compound (section 5) and characteristic (functional) groups, one of which isChemistry Nomenclature Chart
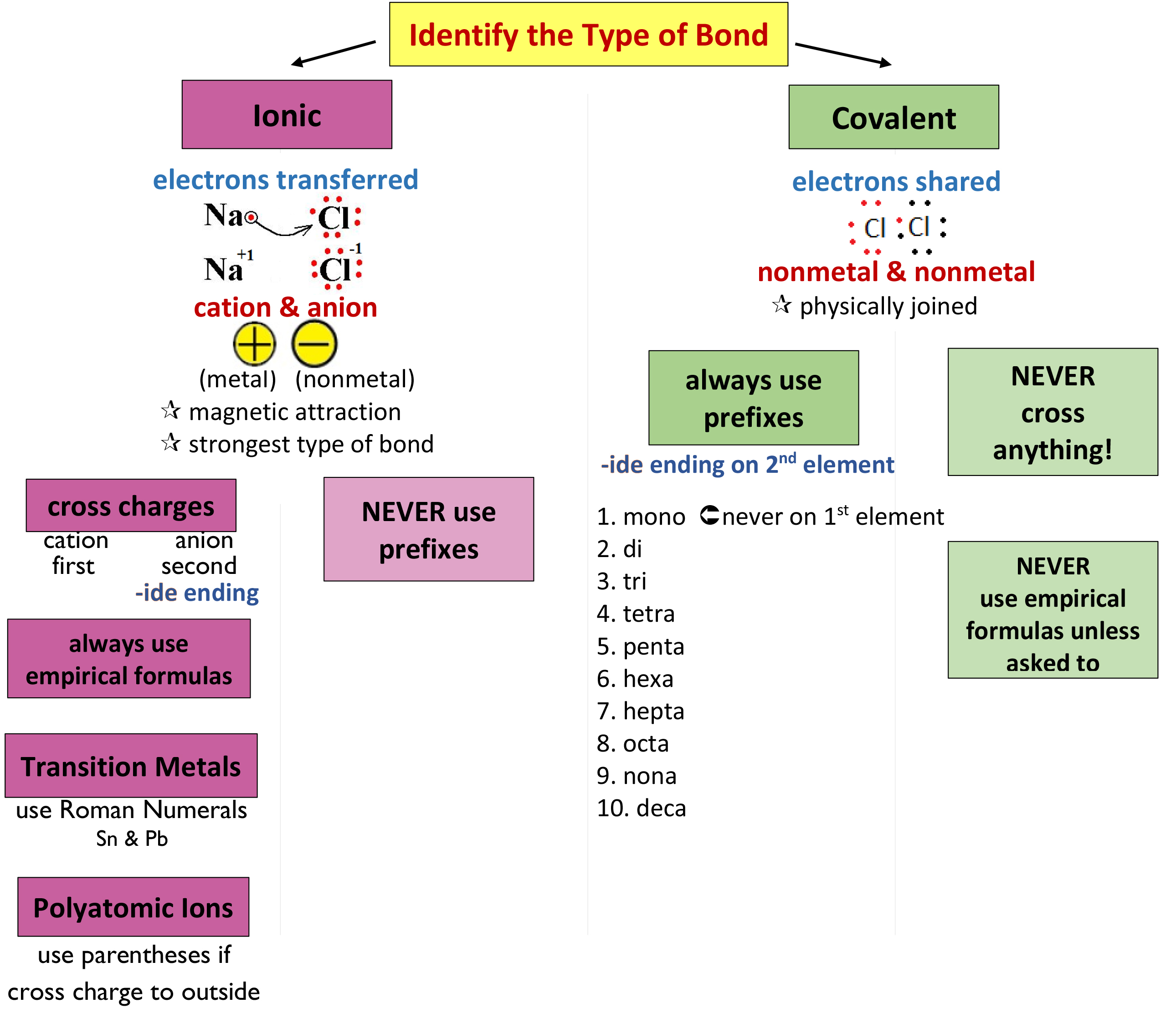
07 Nomenclature Mrs. Cook's Chemistry Class
Chemical Nomenclature and Chemical Formulas Owlcation
Chemistry Nomenclature Chart
Chemistry Nomenclature Chart
CPSC Organic Chemistry Nomenclature
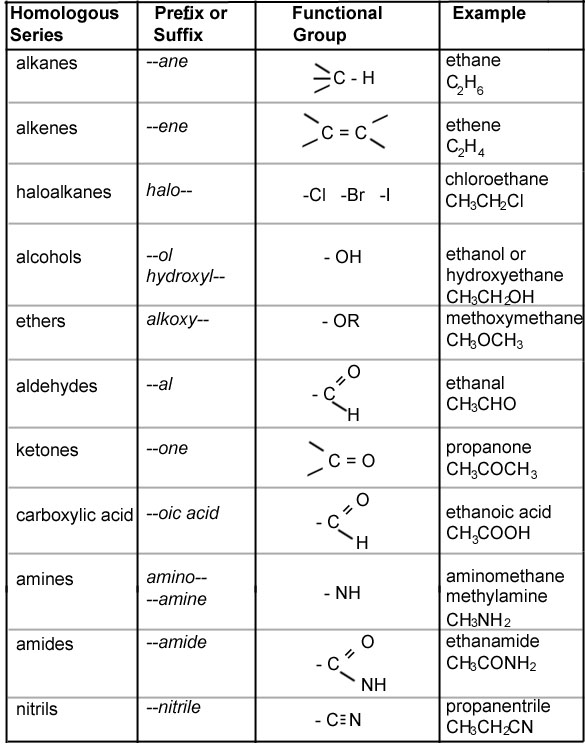
Organic Chemistry Nomenclature Chart
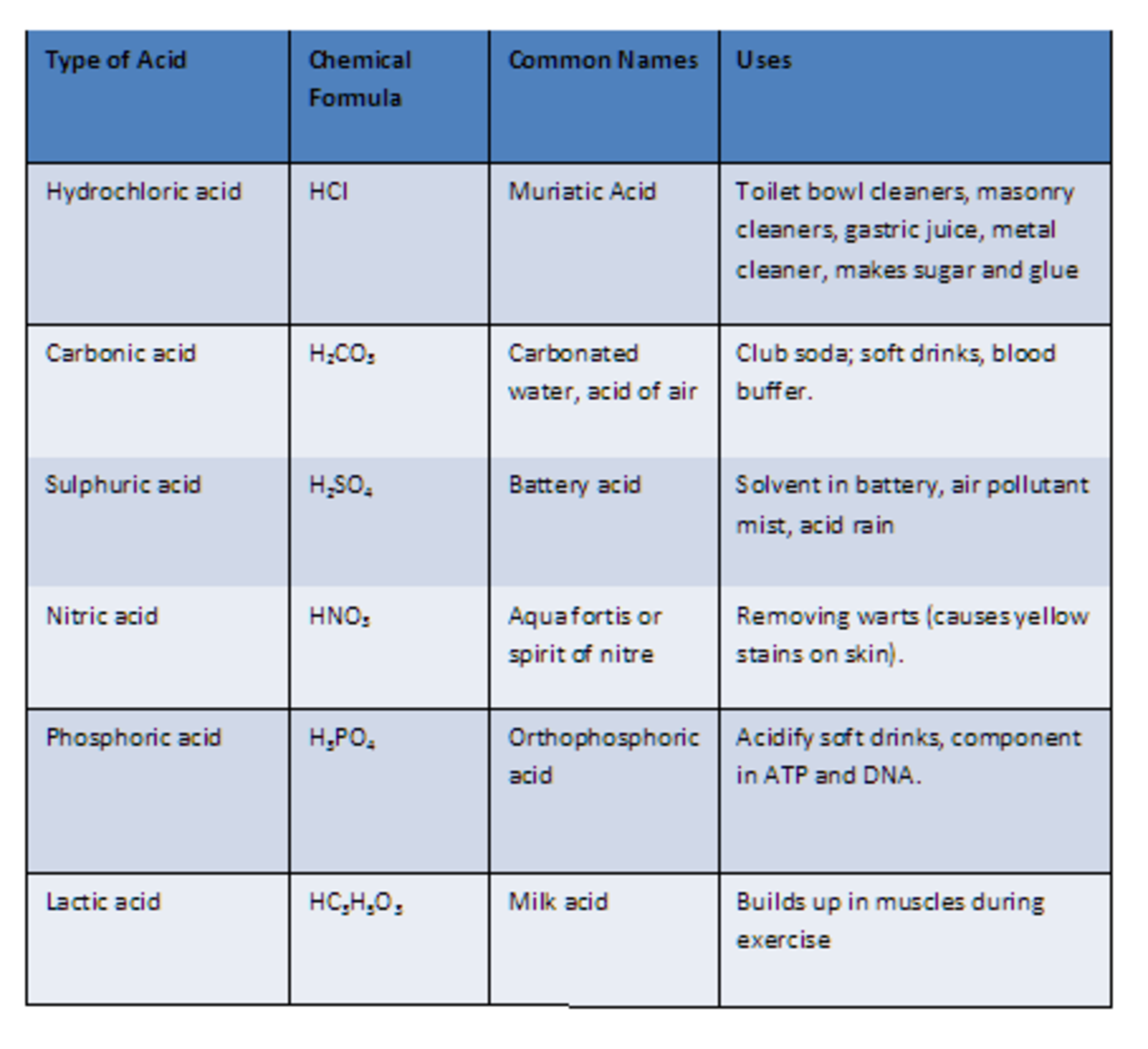
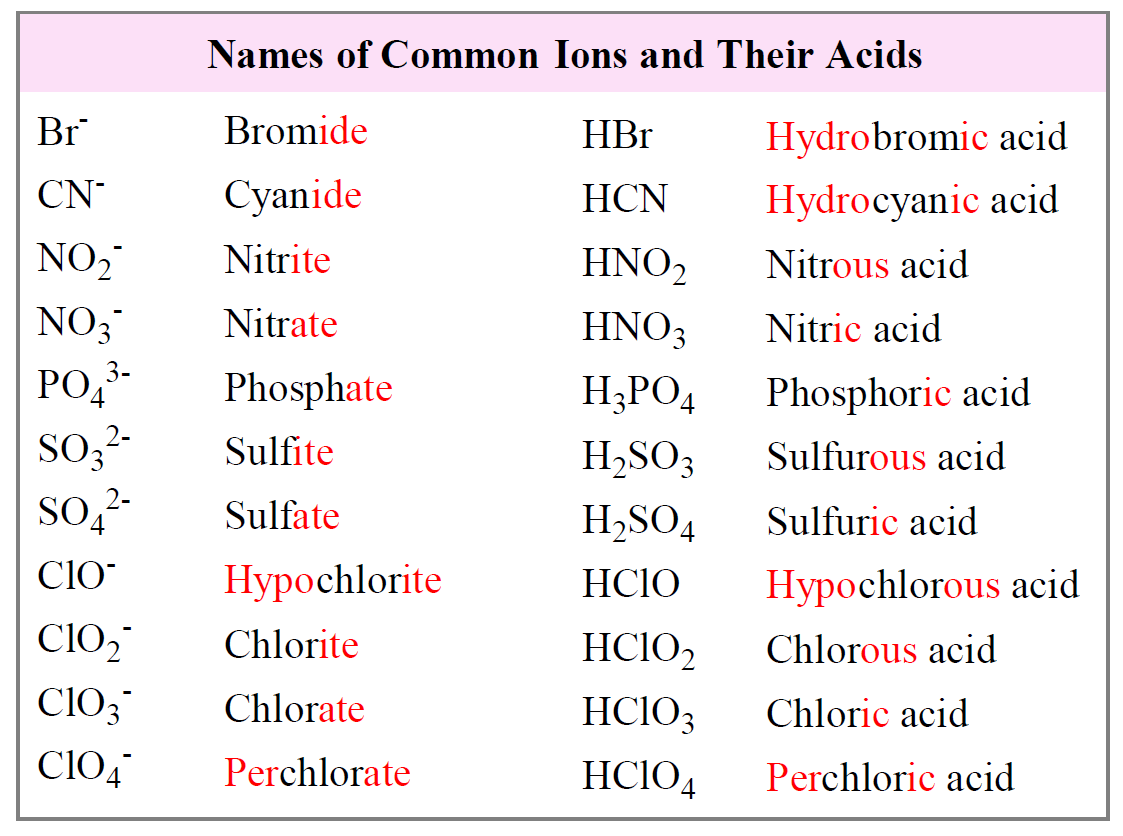
Nomenclature of Acids Pathways to Chemistry
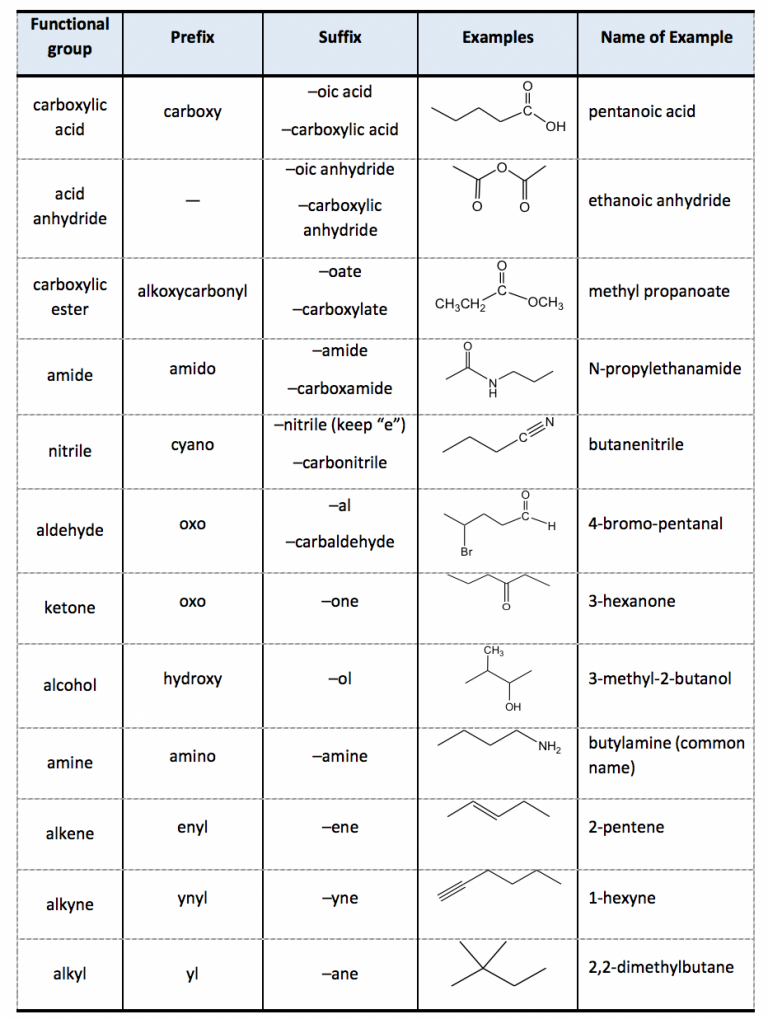
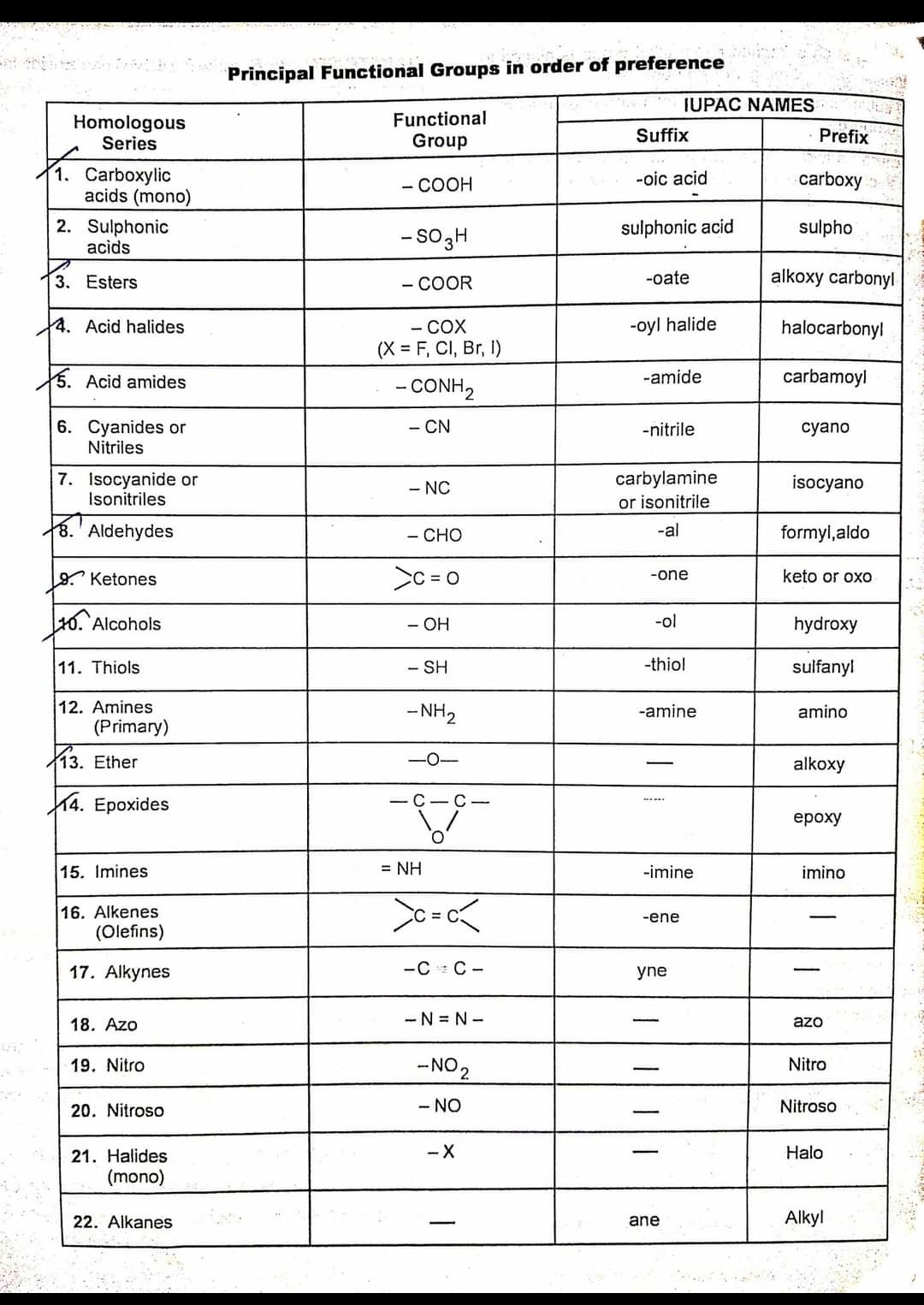
IUPAC Chart Functional Group And Suffix Prefix And Their Principal
Naming Acids and Bases Chemistry Steps
Long Before Chemists Knew The Formulas For Chemical Compounds, They Developed A System Of Nomenclature That Gave Each Compound A Unique Name.
Binary Compound (Metal/Nonmetal) With Fixed Charge Cation.
Web Iupac Nomenclature Is Used For The Naming Of Chemical Compounds, Based On Their Chemical Composition And Their Structure.
Each Chemical Name Should Refer To A Single Substance.
Related Post: