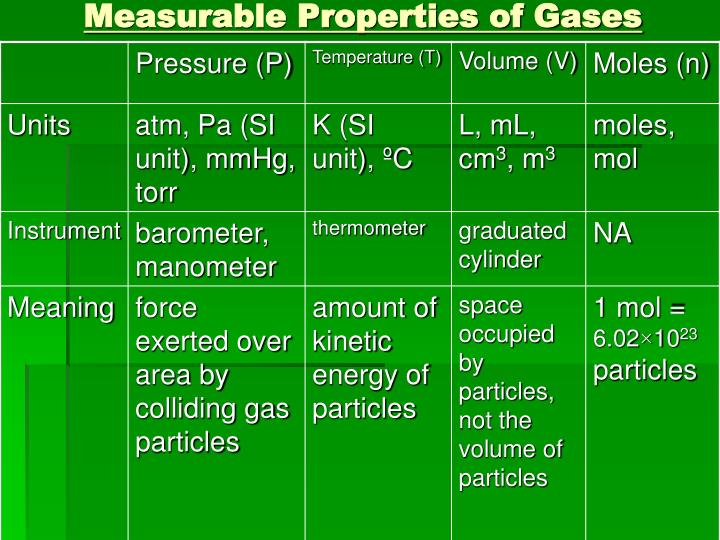
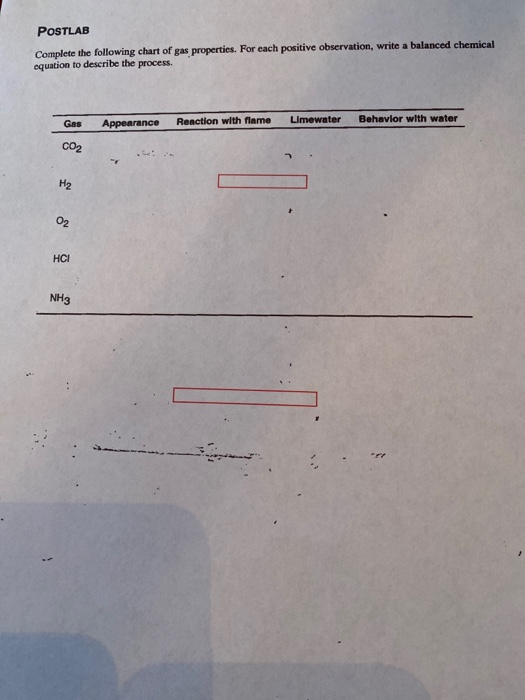
Complete The Following Chart Of Gas Properties For Each Positive
Complete The Following Chart Of Gas Properties For Each Positive - 9.3 stoichiometry of gaseous substances, mixtures, and reactions;. Under standard temperature and pressure (stp, or 1 atm and 273 k), a. Web write a balanced molecular equation describing each of the following chemical reactions. (a) solid calcium carbonate is heated and decomposes to solid. For each positive observation, write a balanced chemical equation to describe the process. Web the temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Web examine the reaction of the gas with lime water to determine if it forms a precipitate indicating the presence of carbon dioxide gas. Web the two most significant properties of noble gases is that they are extremely unreactive, rarely forming compounds, and that they all exist as gases at room temperature. For each positive observation, write a balanced chemical equation to describe the process. gas: Elements that exist as gases at room temperature and. Complete the following chart of gas properties. Web complete the following chart of gas properties. For each positive observation, write a balanced chemical equation to describe the process. gas: For each positive observation, write a balanced chemical equation to describe the process. Web the temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Complete the following tables, showing your work for each lettered box beside the corresponding letter below. For each positive observation, write a balanced chemical equation to describe the process. For each positive equation to describe the process. Unit conversions for the gas laws. Postlab complete the following chart of gas properties. Co2, h2, o2, hcl, nh3 Web write a balanced molecular equation describing each of the following chemical reactions. Web the two most significant properties of noble gases is that they are extremely unreactive, rarely forming compounds, and that they all exist as gases at room temperature. Web your solution’s ready to go! Web 402 rows 9.1 gas pressure; Web write a balanced molecular equation describing each of the following chemical reactions. Postlab complete the following chart of gas properties. Web the two most significant properties of noble gases is that they are extremely unreactive, rarely forming compounds, and that they all exist as gases at room temperature. Postlab equation to describe the process. For each positive observation, write. For each positive equation to describe the process. Web when the properties of the gas (p, v, n, t) are measured under different conditions, these values can be put into the ideal gas equation. Unit conversions for the gas laws. Web 402 rows 9.1 gas pressure; Your solution’s ready to go! Web we will start with covering basic gas properties and then learn several equations of state, which are mathematical equations that relate measurable values like the volume,. (a) solid calcium carbonate is heated and decomposes to solid. Web the temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram. 9.3 stoichiometry of gaseous substances, mixtures, and reactions;. Web the temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Complete the following tables, showing your work for each lettered box beside the corresponding letter below. For each positive observation, write a balanced chemical equation to. Postlab ation, write a balanced chemical complete the following chart of gas properties. Gas, vapour, liquid and solid; Web complete the following chart of gas properties. Under standard temperature and pressure (stp, or 1 atm and 273 k), a. Web 402 rows 9.1 gas pressure; For each positive observation, write a balanced chemical equation to describe the process. Web write a balanced molecular equation describing each of the following chemical reactions. Your solution’s ready to go! For each positive observation, write a balan equation to describe the process. For each positive observation, write a balanced chemical equation to describe the process. gas: Complete the following chart of gas properties. For each positive observation, write a balanced chemical equation to describe the process. For each positive equation to describe the process. Under standard temperature and pressure (stp, or 1 atm and 273 k), a. For each positive observation, write a balanced chemical equation to describe the process. gas: A gas is one of the three classical states of matter (the others being liquid and solid). Elements that exist as gases at room temperature and. 9.2 relating pressure, volume, amount, and temperature: Complete the following chart of gas properties. Web postlab complete the following chart of gas properties. Web make sure you thoroughly understand the following essential ideas: For each positive observation, write a balanced chemical equation to describe the process. Web the temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Unit conversions for the gas laws. (a) solid calcium carbonate is. Web write a balanced molecular equation describing each of the following chemical reactions. Complete the following chart of gas properties. Gas, vapour, liquid and solid; Postlab ation, write a balanced chemical complete the following chart of gas properties. For each positive observation, write a balanced chemical equation to describe the process. gas: For each positive observation, write a balanced chemical equation to describe the process. Web complete the following chart of gas properties. Web examine the reaction of the gas with lime water to determine if it forms a precipitate indicating the presence of carbon dioxide gas. Co2, h2, o2, hcl, nh3 For each positive observation, write a balanced chemical equation to describe the process. Your solution’s ready to go! Elements that exist as gases at room temperature and. Unit conversions for the gas laws. 9.3 stoichiometry of gaseous substances, mixtures, and reactions;. A gas is one of the three classical states of matter (the others being liquid and solid). Web when the properties of the gas (p, v, n, t) are measured under different conditions, these values can be put into the ideal gas equation.Natural gas properties. Download Table
Gases Chart Scholars Labs
PPT Chapter 11 Gases PowerPoint Presentation, free download ID1586364
Worksheet On Ideal Gas Equation Summary Slides Lecture 3 Boyle's
Solved POSTLAB Complete the following chart of gas
Solved Alt Ctrl POSTLAB Complete the following chart of gas
PPT Chapter 12 Section 2 PowerPoint Presentation ID5757546
Properties of Solids, Liquids, and Gases ChemTalk
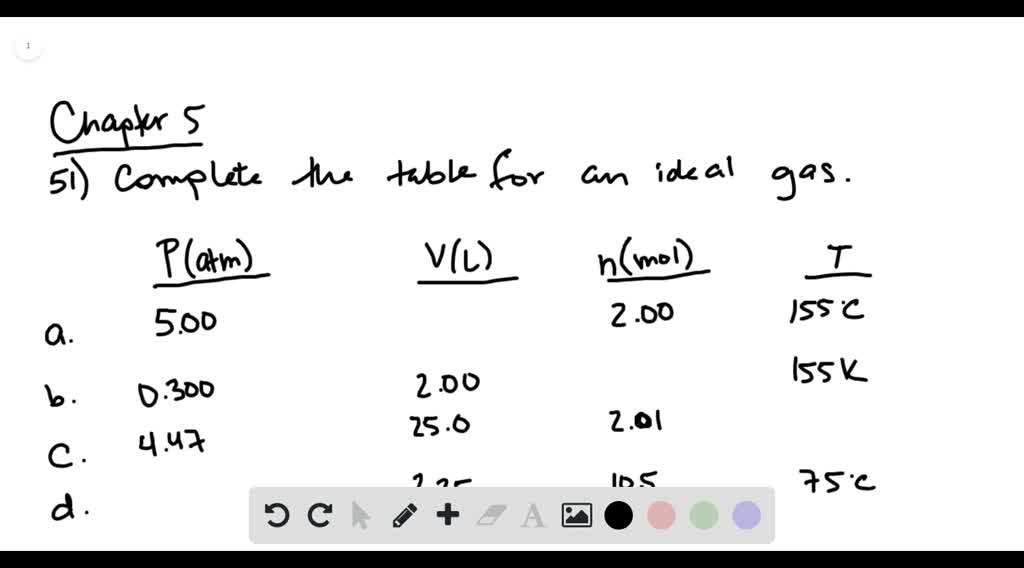
Complete the following table for an ideal gas. (T…
Solved POSTLAB Complete the following chart of gas
For Each Positive Observation, Write A Balan Equation To Describe The Process.
(A) Solid Calcium Carbonate Is Heated And Decomposes To Solid.
9.2 Relating Pressure, Volume, Amount, And Temperature:
Web 402 Rows 9.1 Gas Pressure;
Related Post: