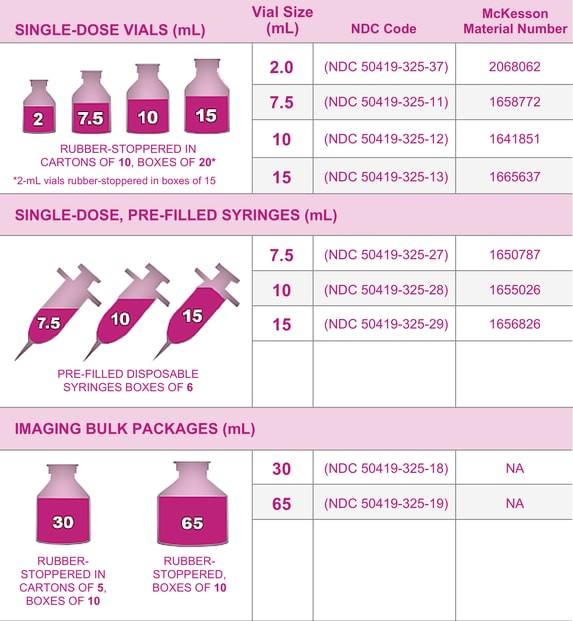
Gadavist Dose Chart
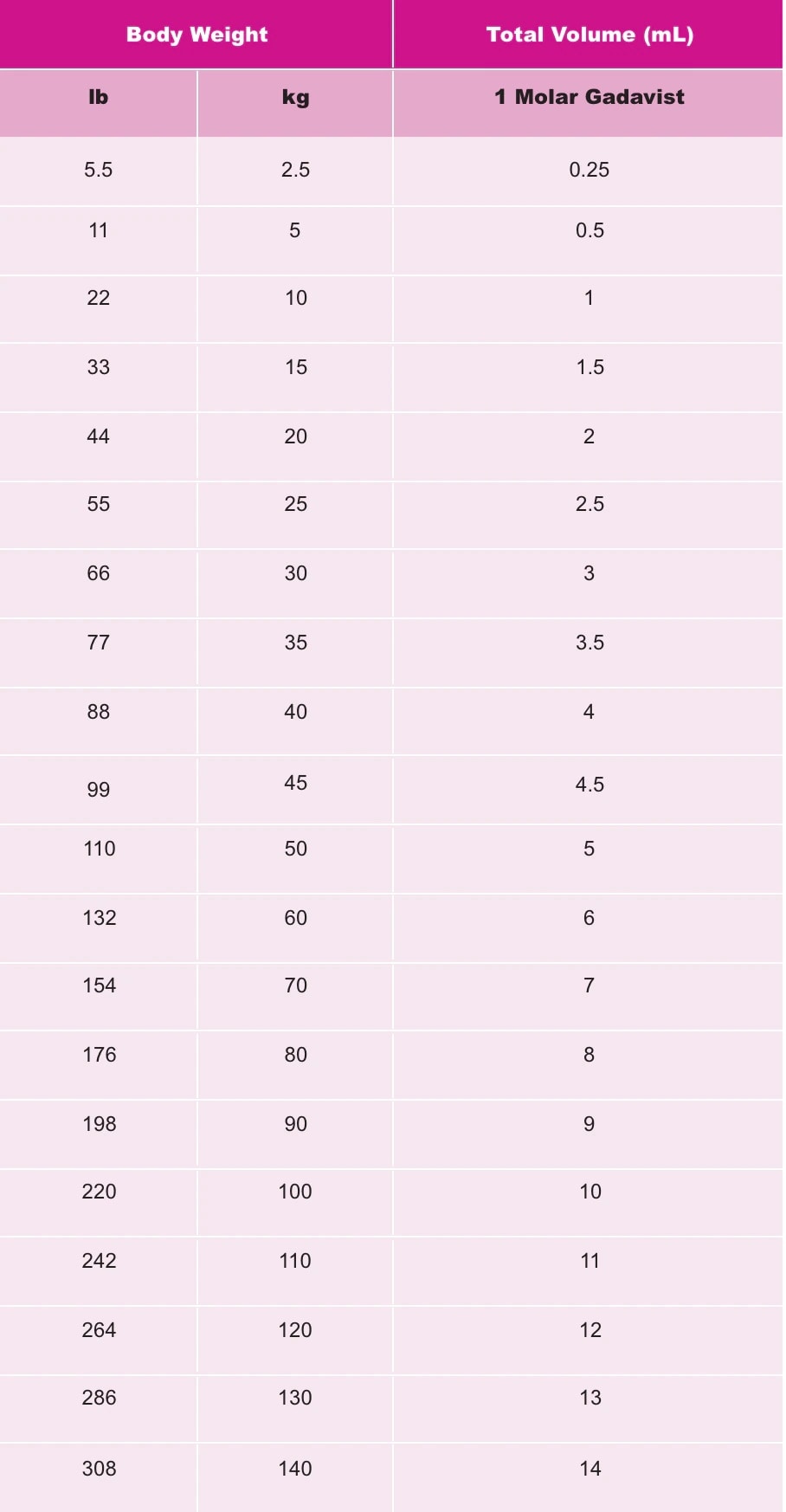
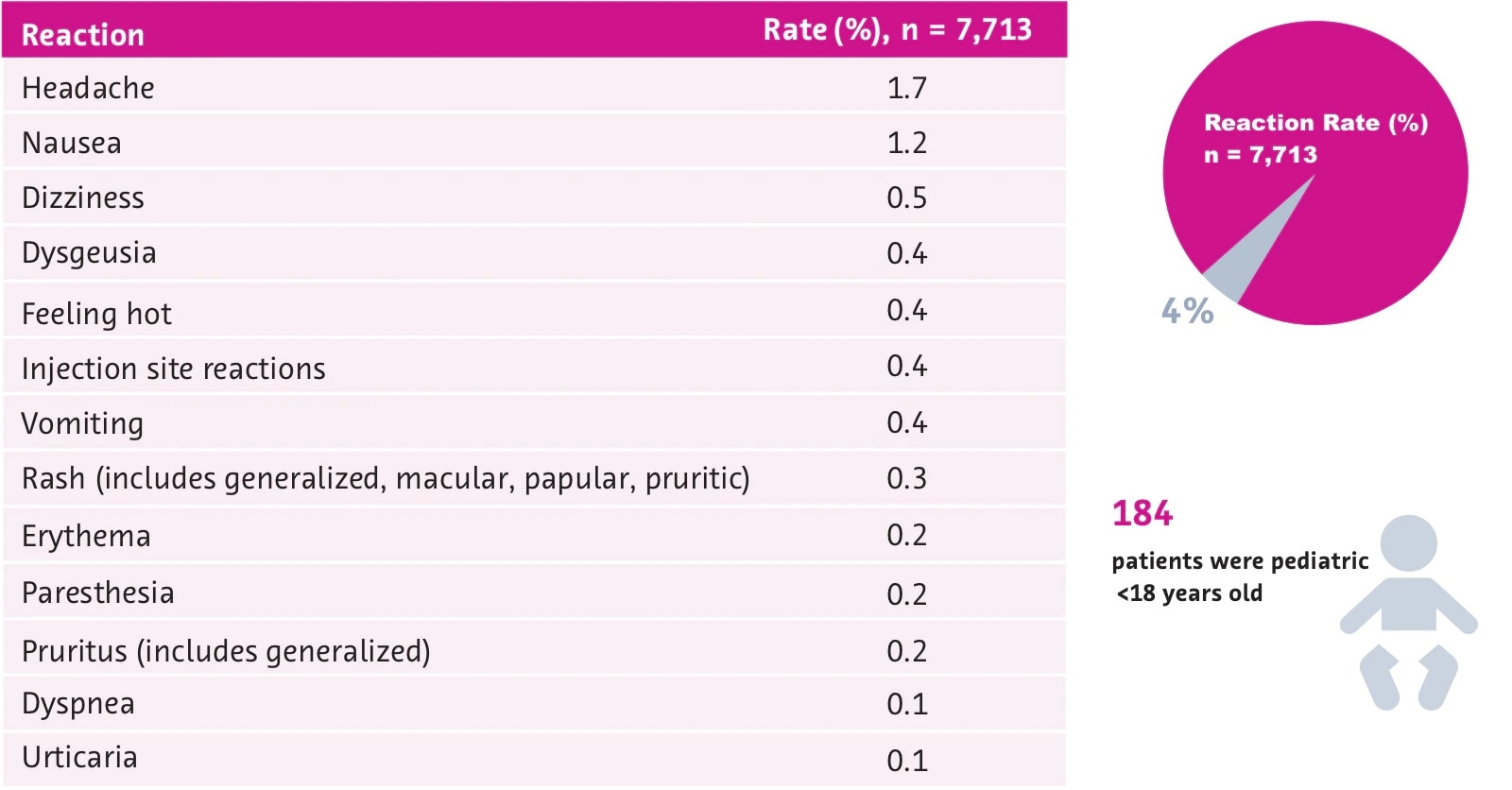
Gadavist Dose Chart - Usual adult dose for cns magnetic resonance imaging: Recommended dosage with gadovist® 1.0 depends on the indication1. 0.1 ml/kg body weight (0.1 mmol/kg) iv bolus injection comments: Refer to table 1 to determine the volume to be. Maximum recommended dose at uw. Gadavist, like other gbcas, is injected into your vein and used with a magnetic resonance imaging (mri) scanner. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Refer to table 1 to determine the volume to. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Administer intravenously as a bolus, manually or by power injector. Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Gadolinium stays in the body more after omniscan or optimark than after eovist, magnevist, or multihance. Refer to table 1 to determine the volume to be. Gadavist, like other gbcas, is injected into your vein and used with a magnetic resonance imaging (mri) scanner. To detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system in adult and pediatric patients including term neonates. Package insert dose of eovist is 0.025mmol/kg. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). Includes dose adjustments, warnings and precautions. Refer to table 1 to determine the volume to be administered. Refer to table 1 to determine the volume to be administered. 0.1 mmol/kg (0.1 ml/kg) iv bolus. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). Refer to table 1 to determine the volume to. Refer to table 1 to determine the volume to be. Maximum recommended dose at uw. Since receiving fda approval in 2011, gadavist ® has received 3 additional indications. Gadavist, like other gbcas, is injected into your vein and used with a magnetic resonance imaging (mri) scanner. Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Refer to table 1 to determine. Web dosage for gadavist recommended dose. 0.1 ml/kg) administered intravenously at approximately 2 ml/sec. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Web the recommended. Administer manually or by power injector at a flow rate of approximately 2 ml/second. Web dosage for gadavist recommended dose. Refer to manufacturer product information to determine volume to be administered. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Includes dose adjustments, warnings and precautions. Refer to table 1 to determine the volume to. Administer intravenously as a bolus, manually or by power injector. 0.1 ml/kg) administered intravenously at approximately 2 ml/sec. (0.1 mmol/kg body weight) is sufficient to answer the clinical questions.1. Gadavist, like other gbcas, is injected into your vein and used with a magnetic resonance imaging (mri) scanner. Refer to table 1 to determine the volume to be administered. 1mmol/ml (ie, 604.72mg/ml) mri contrast. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Volume of gadavist injection by body weight* Refer to table 1 to determine the volume to. Refer to table 1 to determine the volume to be. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Refer to manufacturer product information to determine volume to be administered. Web the recommended dose of gadavist for adult and pediatric patients. Web detailed dosage guidelines and administration information for gadavist (gadobutrol). Refer to table 1 to determine the volume to be. Volume of gadavist injection by body weight* Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Note maximum dose of eovist is 0.05mmol/kg. Administer intravenously as a bolus, manually or by power injector. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Refer to manufacturer product information to determine volume to be administered. 0.1 ml/kg body weight (0.1 mmol/kg) iv bolus injection comments: Web. Generally, a single injection of 0.1 ml/kg body weight. Usual adult dose for cns magnetic resonance imaging: 1mmol/ml (ie, 604.72mg/ml) mri contrast. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Web the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an. Administer manually or by power injector at a flow rate of approximately 2 ml/second. Web detailed dosage guidelines and administration information for gadavist (gadobutrol). Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Since receiving fda approval in 2011, gadavist ® has received 3 additional indications. Refer to table 1 to determine the volume to be administered. Package insert dose of eovist is 0.025mmol/kg. Generally, a single injection of 0.1 ml/kg body weight. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Gadolinium stays in the body the least after dotarem, gadavist, or prohance. 0.1 mmol/kg (0.1 ml/kg) iv bolus. Includes dose adjustments, warnings and precautions. Refer to table 1 to determine the volume to be. Web at equivalent doses, the amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after omniscan or optimark than after eovist, magnevist, or multihance. Volume of gadavist injection by body weight* Refer to table 1 to determine the volume to.Gadavist (gadobutrol) injection Radiology US
Stability of Gadolinium Based Contrast Agents (GBCAs)
Gadavist Contrast Media Gadobutrol 1 mmol / mL Intravenous Injection
Gadavist (gadobutrol) injection Radiology US
Gadavist (gadobutrol) injection Radiology US
Gadavist™ Gadobutrol 1 mmol / mL Injection 10 mL McKesson
Gadavist (gadobutrol) injection Radiology US
Medrad, NDC 5041932513, Bayer Gadavist (Gadobutrol) Inje... eSutures
Clinical and biochemical characteristics of LADA patients at the time
To Detect And Visualize Areas With Disrupted Blood Brain Barrier And/Or Abnormal Vascularity Of The Central Nervous System In Adult And Pediatric Patients Including Term Neonates.
For Patients At Risk For Chronically Reduced Renal Function (For Example, Age >60 Years, Hypertension Or Diabetes), Estimate The Glomerular Filtration Rate.
Web Gadolinium Based Contrast Dosing Charts:
The Recommended Dose Of Gadavist For Adult And Pediatric Patients (Including Term Neonates) Is 0.1 Ml/Kg Body Weight Mmol/Kg).
Related Post: