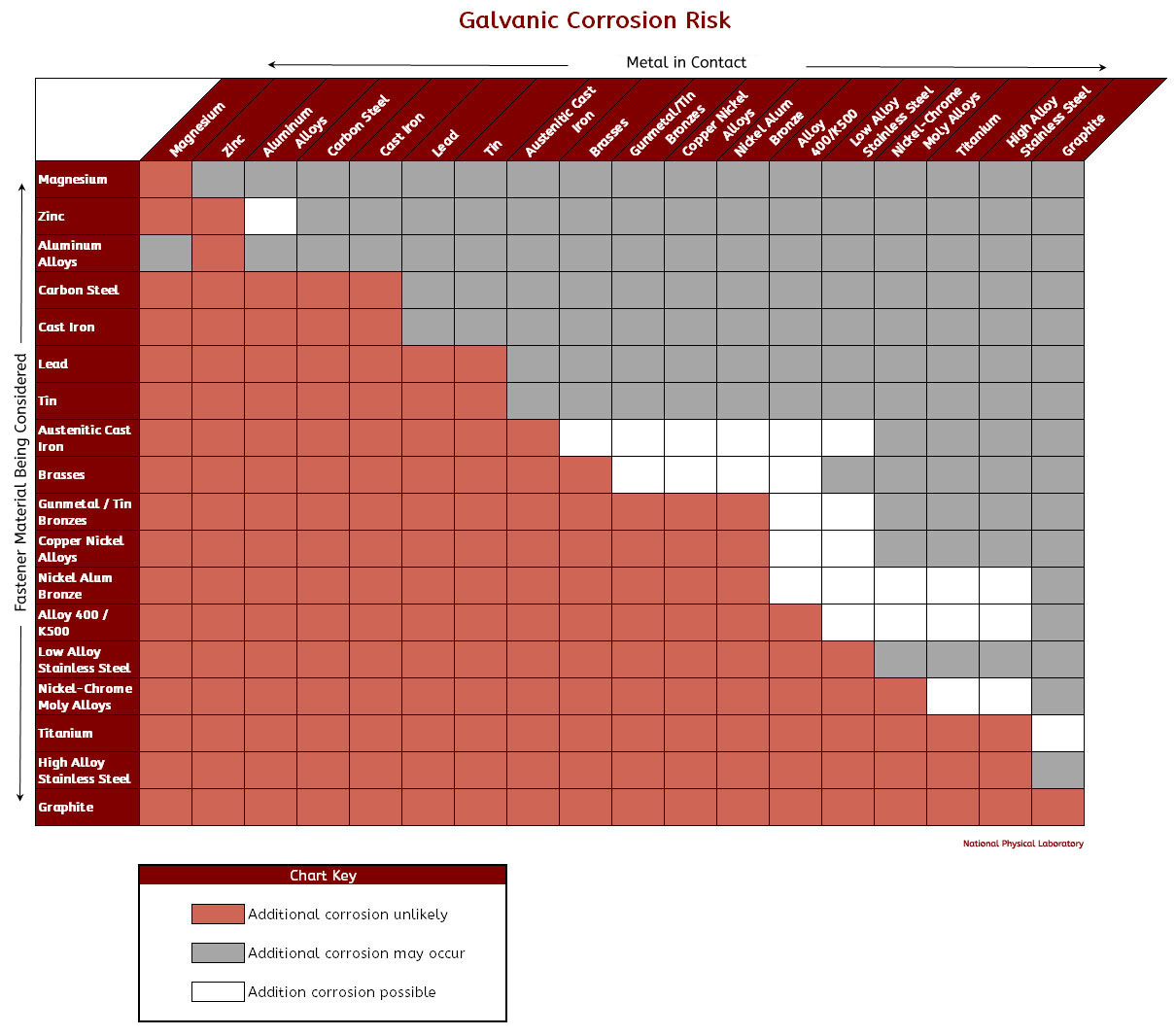
Galvanic Chart Corrosion
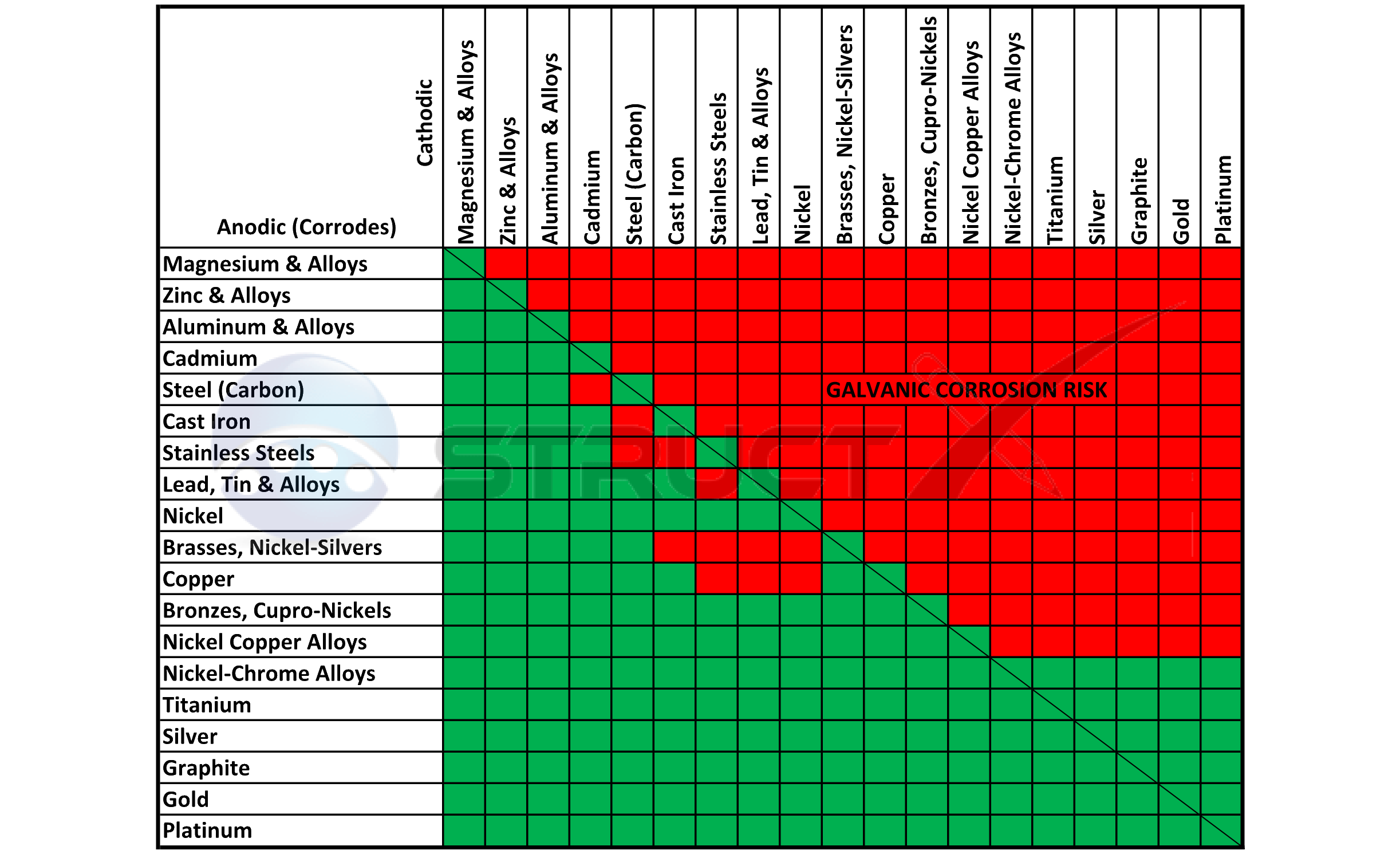
Galvanic Chart Corrosion - Web below is a galvanic reaction chart for dissimilar metals. This can help you in the selection of the best. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web galvanic corrosion is of particular concern in design and material selection. Fastened of galvanic corrosion in joint, it’s. Web the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. The most active metals in the galvanic corrosion chart, like aluminum, zinc, or. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. A typical rule of thumb is that. A typical rule of thumb is that. Web the galvanic corrosion table ranks metals from the most “active” to the least active. Material selection is important because different metals come into contact with each other and. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. Web below is a galvanic reaction chart for dissimilar metals. Web below is a galvanic reaction chart for dissimilar metals. Web below is the galvanic corrosion chart, along with steps for using the chart properly to determine metal compatibility. Web there are four elements necessary for corrosion to occur in a galvanic cell: This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. A typical rule of thumb is that. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. We consider two metals compatible if their emf. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when. Web below is a galvanic reaction chart for dissimilar metals. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Web typically, the presence of an electrolyte (eg. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web below, we give a brief overview of. Web by knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic. The closer together the material are on the chart to the right, the less. Zinc coatings on carbon steel and. Web below is a galvanic reaction chart for dissimilar metals. Material selection is important because different metals. Web when corrosion does occur, the anodic material is the most likely to corrode, whereas the cathodic material is the least likely to corrode. Web typically, the presence of an electrolyte (eg. Web below is the galvanic corrosion chart, along with steps for using the chart properly to determine metal compatibility. This chart is designed to assist in broadly assessing. Web galvanic corrosion is of particular concern in design and material selection. Web below is a galvanic reaction chart for dissimilar metals. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the. Material selection is important because different metals come into contact with each other and. Fastened of galvanic corrosion in joint, it’s. However, understanding how it works and the steps that can be taken to protect against. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). The larger the separation distance. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A typical rule of thumb is that. However, understanding how it works and the steps that can be taken to protect against. The most active metals. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web the metal with the higher potential forms the anode and is corroded. The larger the separation distance in the electromotive chart between the two metals. This can help you in the selection of the best. Web there are four elements necessary for corrosion to occur in a galvanic cell: Web by knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic. However, understanding how it works and the steps that can be taken to. Web below is a galvanic reaction chart for dissimilar metals. Web the galvanic corrosion table ranks metals from the most “active” to the least active. This can help you in the selection of the best. The larger the separation distance in the electromotive chart between the two metals in contact, the. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. The most active metals in the galvanic corrosion chart, like aluminum, zinc, or. Web by knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic. For any combination of dissimilar metals, the metal with the lower number will act. Zinc coatings on carbon steel and. Web the metal with the higher potential forms the anode and is corroded. The below galvanic corrion chart or anodic index table shows anodic index for different materials. This phenomenon is named after italian ph… Web there are four elements necessary for corrosion to occur in a galvanic cell: Web below is the galvanic corrosion chart, along with steps for using the chart properly to determine metal compatibility. Material selection is important because different metals come into contact with each other and. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming.Galvanic Corrosion Chart Metals
Galvanic Corrosion CMP Products Limited
Galvanic Corrosion Common Questions Answered
Galvanic Corrosion SSINA
Galvanic Action Corrosion Prevention Architect's Blog
Galvanic Series (electrochemical series)
Galvanic Corrosion [with Chart] EngineerExcel
Galvanic Corrosion Chart PDF Corrosion Electrochemistry
GALVANICCORROSIONTABLE CMP Products Limited
Web Galvanic Corrosion Is Of Particular Concern In Design And Material Selection.
Fastened Of Galvanic Corrosion In Joint, It’s.
Web Typically, The Presence Of An Electrolyte (Eg.
Web Galvanic Corrosion (Some Times Called Dissimilar Metal Corrosion) Is The Process By Which The Materials In Contact With Each Other Oxidizes Or Corrodes.
Related Post:
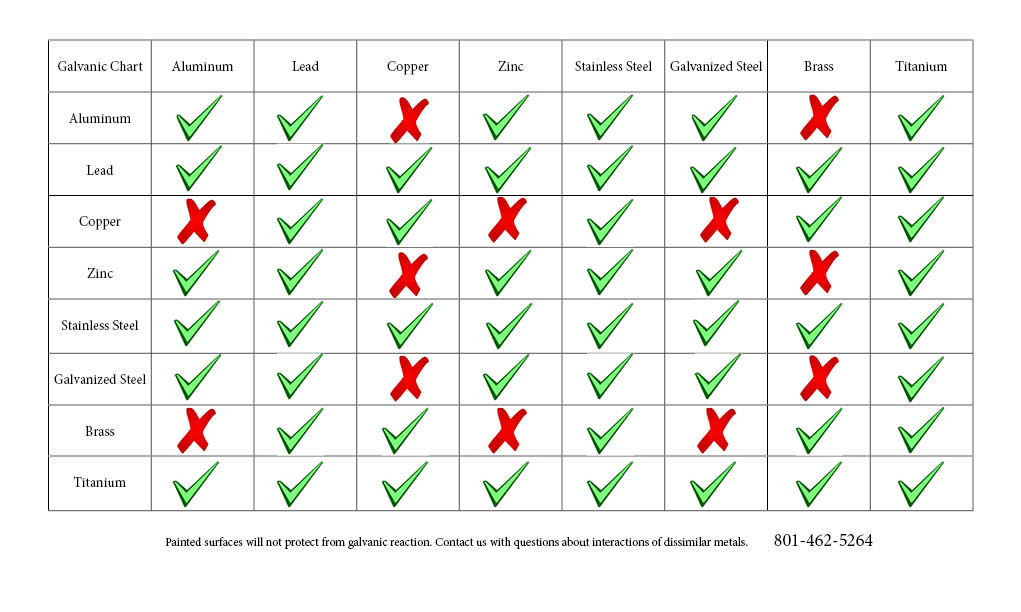

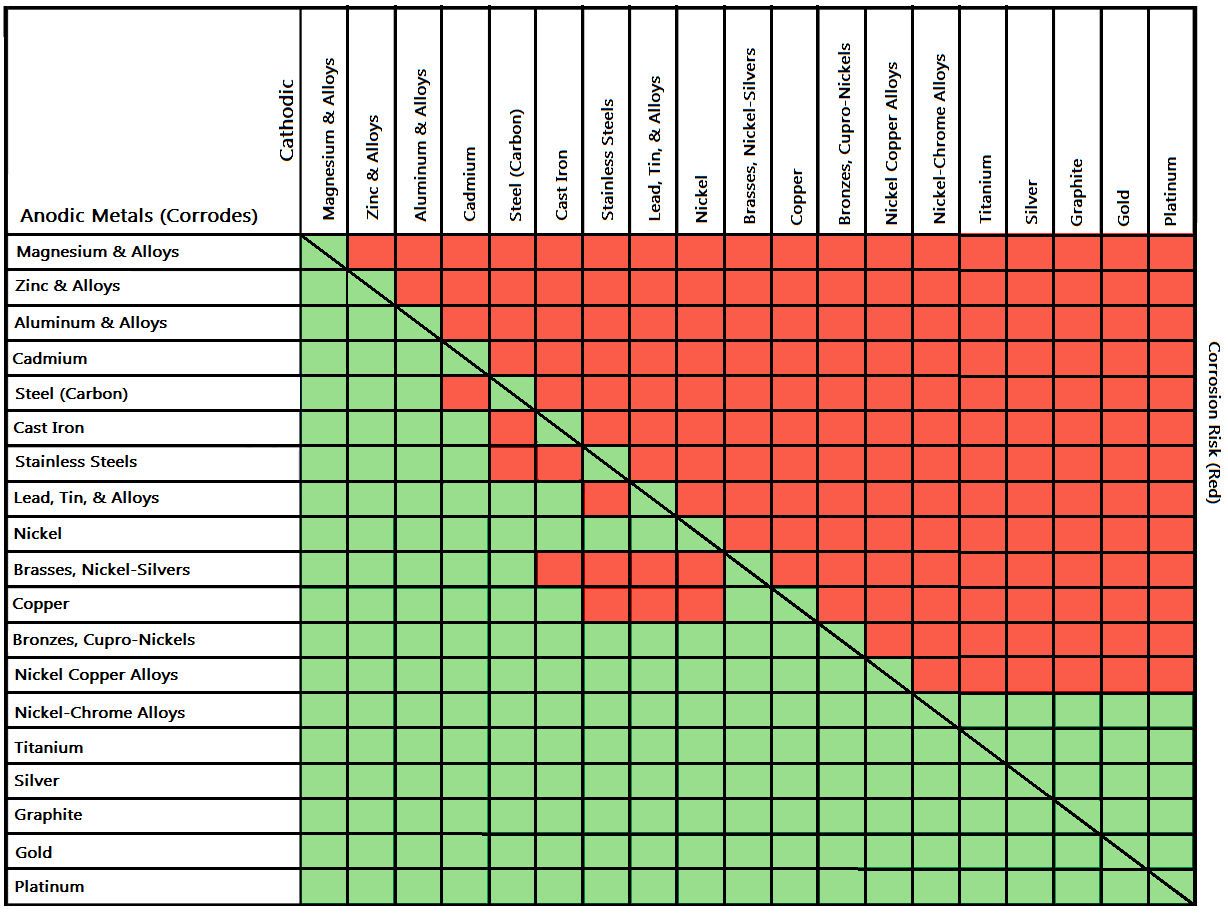
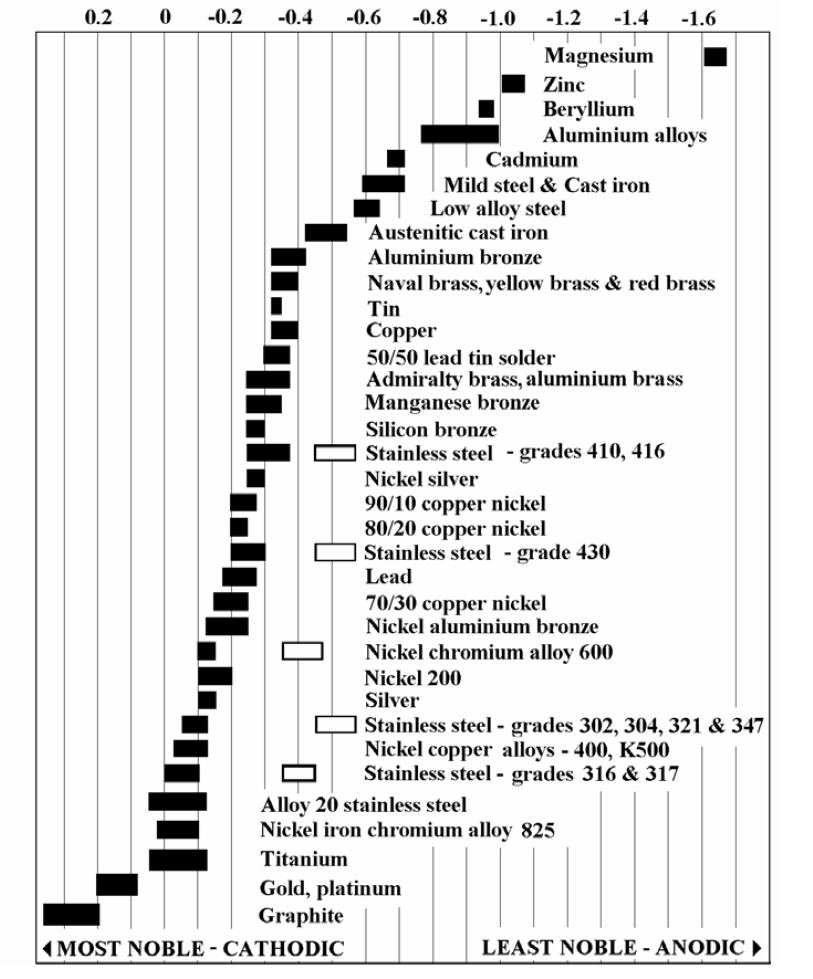

![Galvanic Corrosion [with Chart] EngineerExcel](https://engineerexcel.com/wp-content/uploads/2023/03/galvanic-corrosion-chart.png)