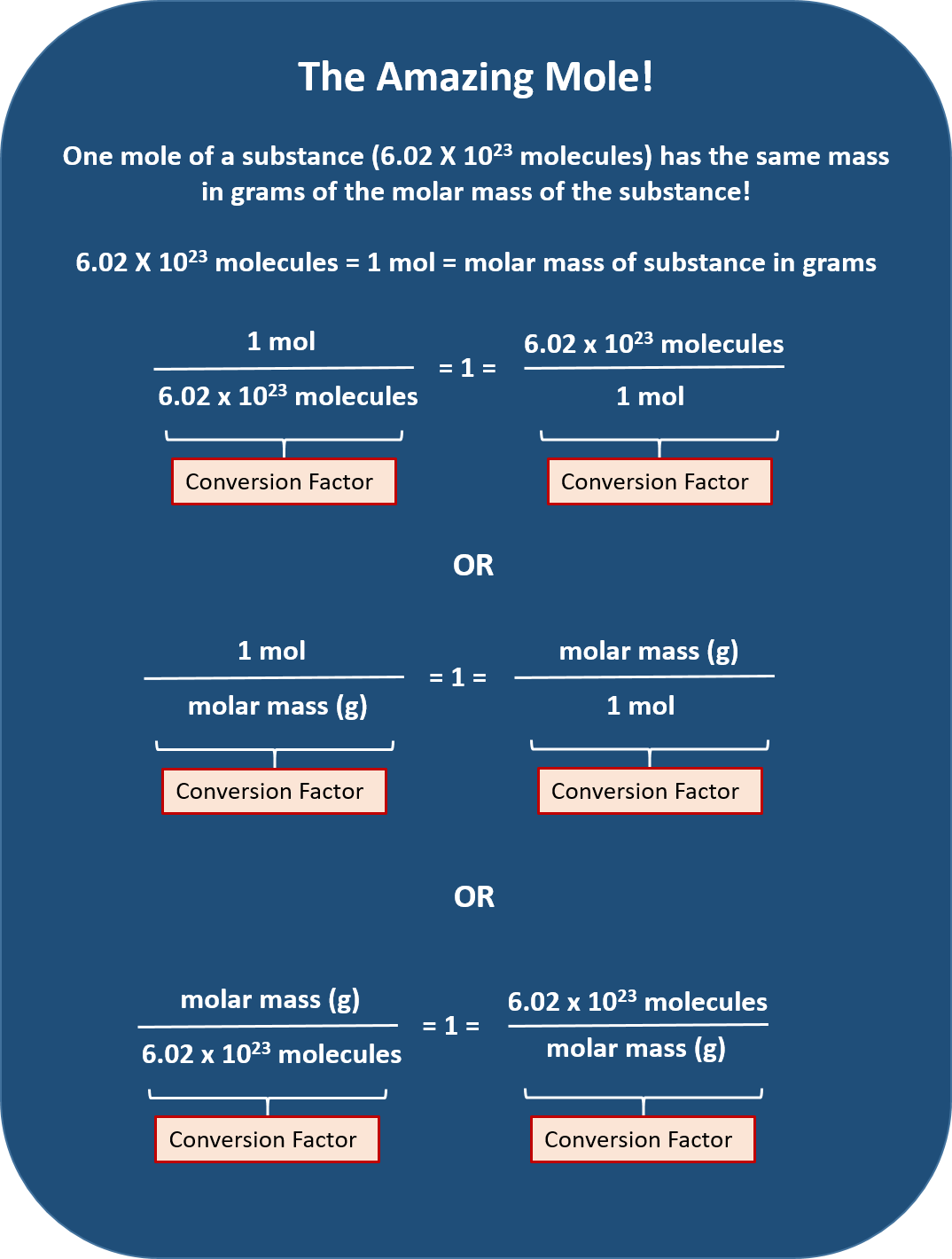
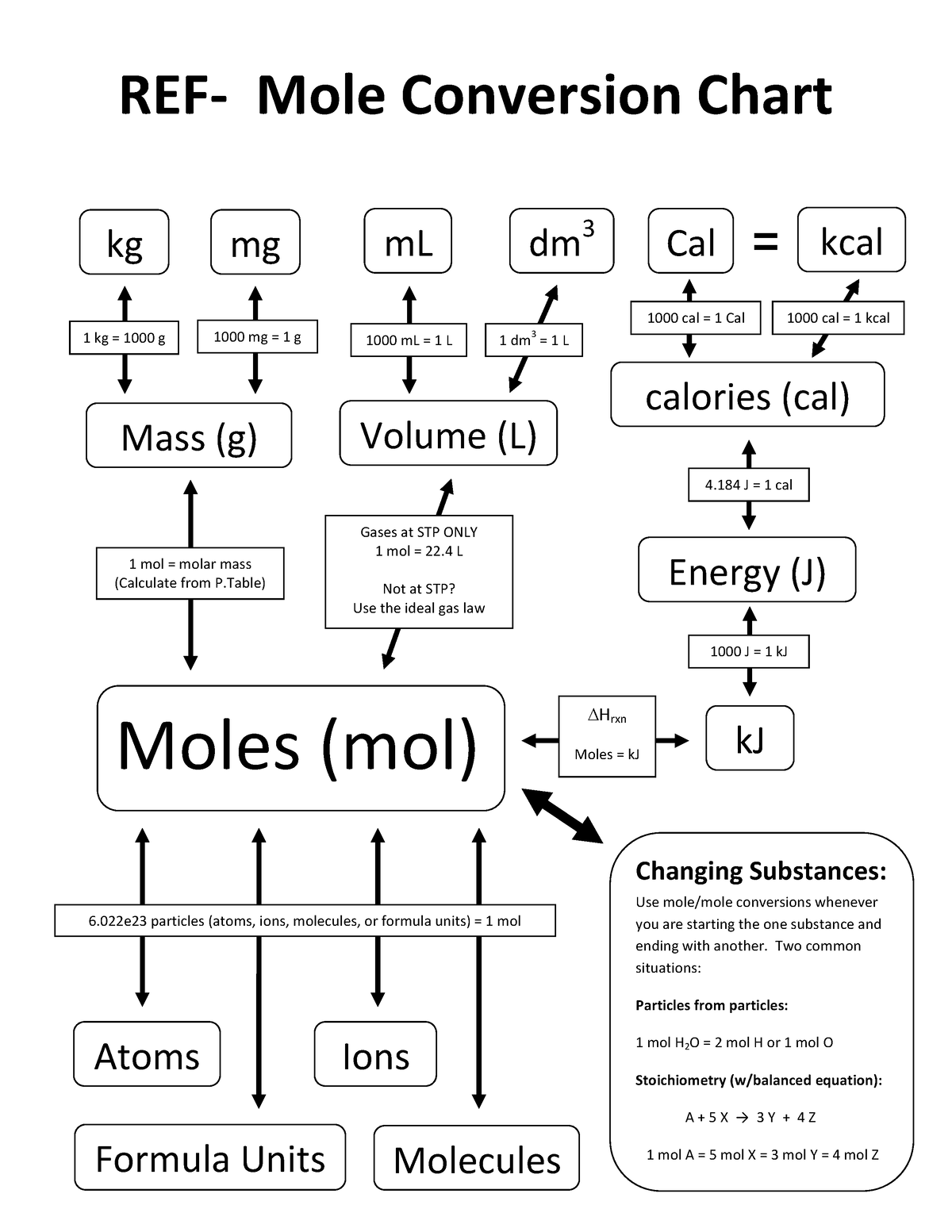
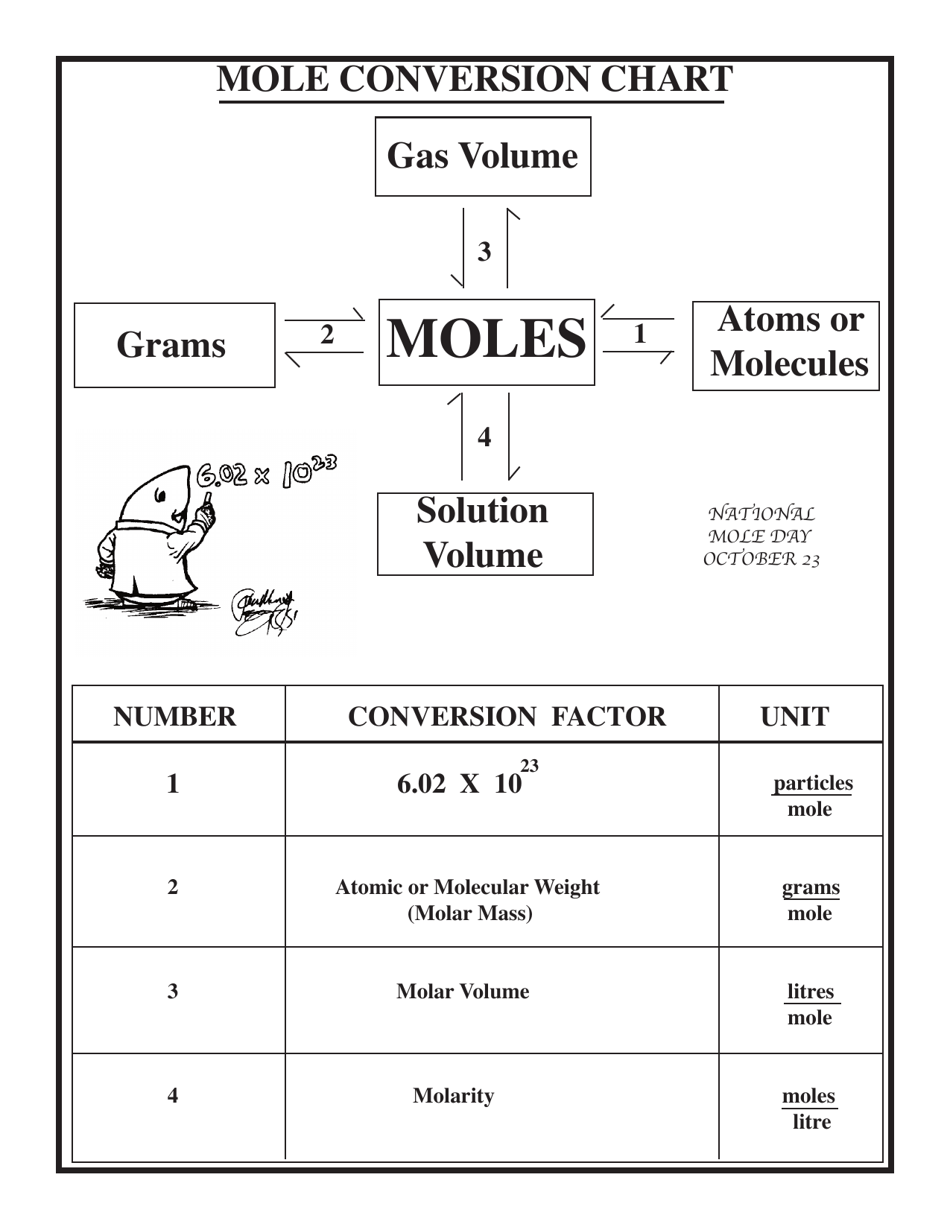
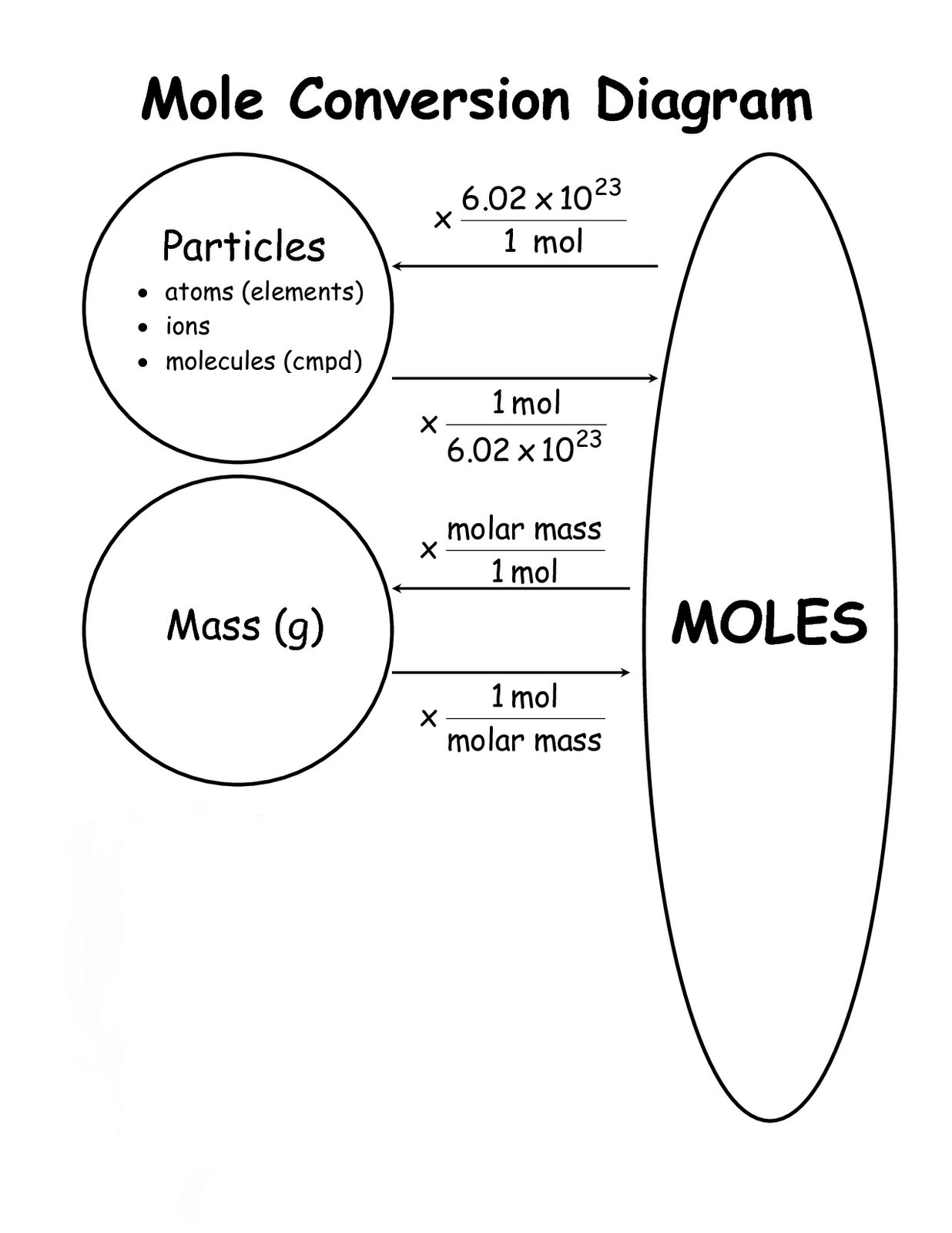
Mole Conversions Chart
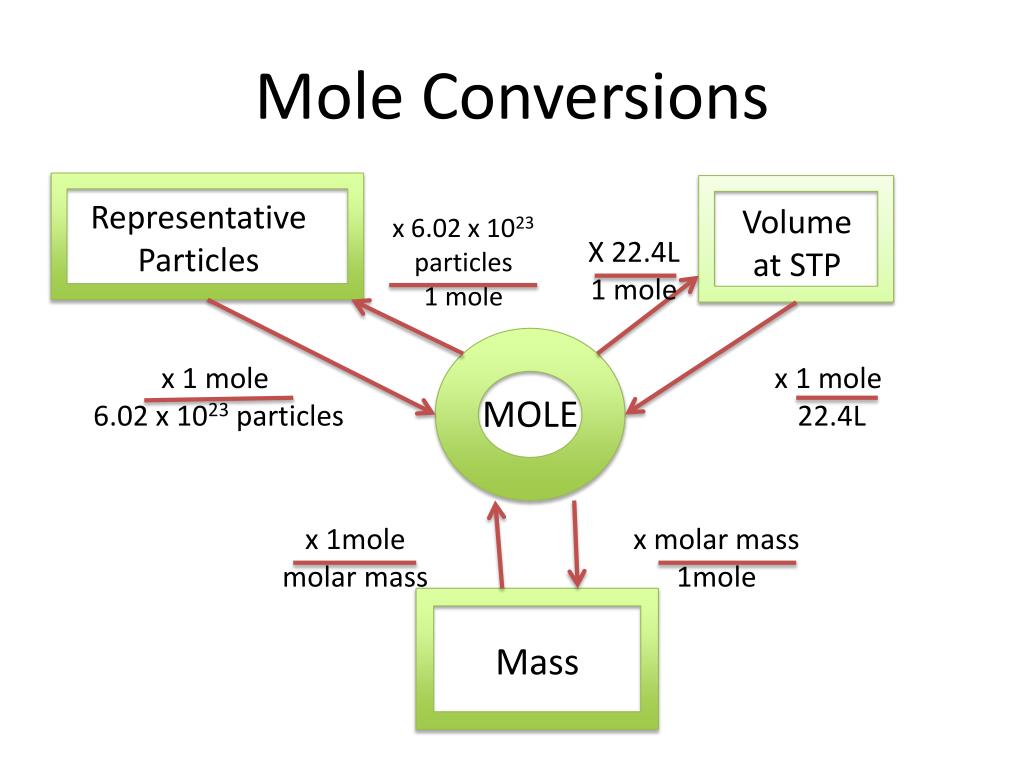
Mole Conversions Chart - How many moles of salt are in 13.8 g of sodium chloride? Using our unit conversion techniques, we can use the mole label to convert back and forth between the number. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. Multiply your mole value by the molar volume constant, 22.4l. You are only allowed to go from left to right. Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole. Express the answer using 3. Web how is the molarity of a percentage solution calculated? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Web enter the molecular formula of the substance. The study of the numerical. Depending on the input data it can serve either as grams to moles. Multiply your mole value by the molar volume constant, 22.4l. 70% nitric acid means that 100 grams of this acid contains 70 grams of. Convert from mass or moles of one substance to mass or moles of another substance in a. Web in this video you'll learn to use the mole map to visually build a plan to convert from moles to grams with six typical mole to grams conversion problems. How many moles of salt are in 13.8 g of sodium chloride? Converting from particles (atoms, molecules, or formula. Web this mole calculator helps to determine the number of moles, mass, or molecular weight of a substance. Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole. Our moles to grams converter makes it easy to convert between molecular weight, mass, and moles. Depending on the input data it can serve either as grams. Depending on the input data it can serve either as grams to moles. Web the calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Web enter the molecular formula of the substance. Using our unit conversion techniques, we can use the mole label to convert back and forth between the number.. Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole. Web conversions between moles and number of particles. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction.. Web in this video you'll learn to use the mole map to visually build a plan to convert from moles to grams with six typical mole to grams conversion problems. Web you might be wondering how you can calculate the number of moles given a mass, volume, or amount of substance. Convert from mass or moles of one substance to. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Web conversions between moles and number of particles. Follow along, and we’ll show you how to convert mass to. Web mole conversion chart national mole day october 23. Using our unit conversion techniques, we can use the mole label to convert back and forth between the number. Web perform conversions between mass and moles of a substance. 70% nitric acid means that 100 grams of this acid contains 70 grams of. You are only allowed to go from left to right. The study of the numerical. Converting from particles (atoms, molecules, or formula. Web this mole calculator helps to determine the number of moles, mass, or molecular weight of a substance. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. It will calculate the total mass along with the elemental composition and mass of each element in the compound. Web conversions like this are possible for any substance, as. Web mole conversion chart national mole day october 23. Web moles are a type of unit conversion used in chemistry to measure the amount of substances. The great divide side, you will divide. Convert from mass or moles of one substance to mass or moles of another substance in a. Web converting from moles to volume (liters): To help you remember how to convert between moles, volume at stp, mass, and number of particles, we made this simple visual. Convert from mass or moles of one substance to mass or moles of another substance in a. Web moles are a type of unit conversion used in chemistry to measure the amount of substances. The molecular weight of. The great divide side, you will divide. Convert from mass or moles of one substance to mass or moles of another substance in a. Web this mole calculator helps to determine the number of moles, mass, or molecular weight of a substance. Express the answer using 3. You are only allowed to go from left to right. You are only allowed to go from left to right. Convert grams to moles effortlessly with this tool. 70% nitric acid means that 100 grams of this acid contains 70 grams of. Express the answer using 3. Web perform conversions between mass and moles of a substance. Using our unit conversion techniques, we can use the mole label to convert back and forth between the number. Web converting from moles to volume (liters): The great divide side, you will divide. Using 70% concentrated nitric acid as an example: The study of the numerical. Web you might be wondering how you can calculate the number of moles given a mass, volume, or amount of substance. Web moles are a type of unit conversion used in chemistry to measure the amount of substances. Web enter the molecular formula of the substance. Web the calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and. Our moles to grams converter makes it easy to convert between molecular weight, mass, and moles.Chapter 6 Quantities in Chemical Reactions Chemistry
Mole Conversion Chart Basicand Advanced Conversions1 REF Mole
Chemistry Cheat Sheet Mole Conversion Chart Download Printable PDF
Mole Conversion Chart
Chemistry Mysteries Mole Conversions
Mole Conversion Cheat Sheet
Chemistry Conversion Chart Moles
Molar Mass Conversion Chart
Chemistry Conversion Chart Moles
Chemistry Mole Conversion Chart
Web Figure \(\Pageindex{1}\) Is A Chart For Determining What Conversion Factor Is Needed, And Figure \(\Pageindex{2}\) Is A Flow Diagram For The Steps Needed To.
Follow Along, And We’ll Show You How To Convert Mass To.
Multiply Your Mole Value By The Molar Volume Constant, 22.4L.
Depending On The Input Data It Can Serve Either As Grams To Moles.
Related Post: