Vapor Pressure Chart Water
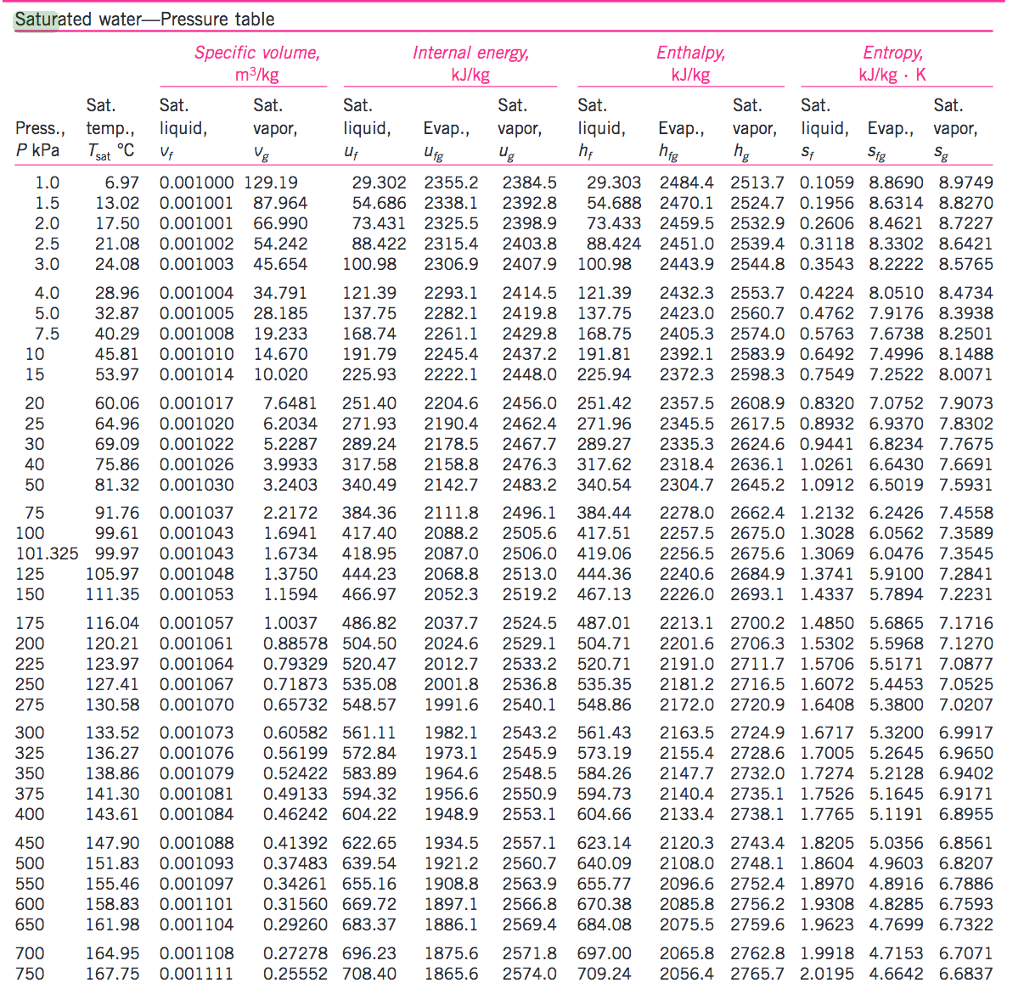
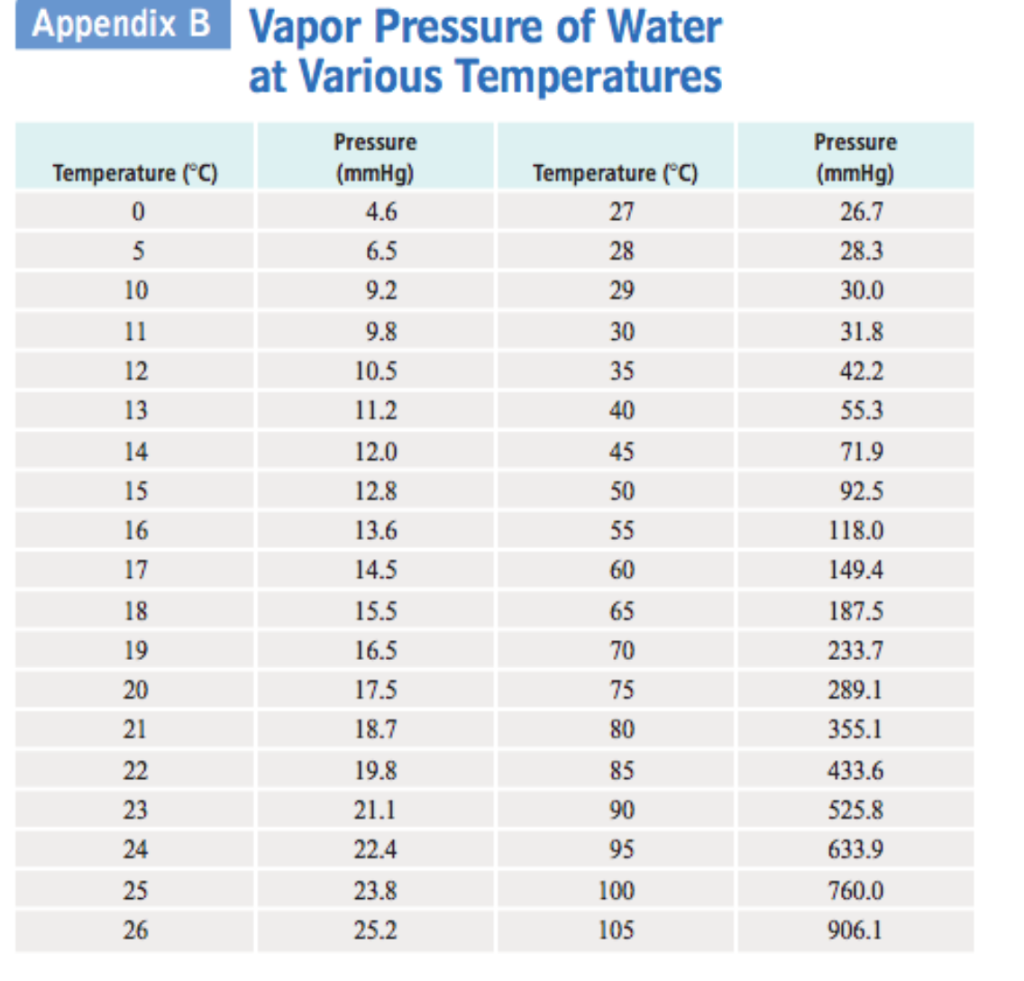
Vapor Pressure Chart Water - Crc handbook of chemistry and physics, 84th edition (2004). Web vapor pressure of water from 0 °c to 100 °c. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa. It is the pressure exerted by the saturated vapour in contact with the surface. The output temperature is given as °c, °f, k and °r. Web vapor pressure of water (mmhg) source of data: Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. The vapor pressure of pure water is 47.1. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the. Vapor pressure of water is shared under a not. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the. Web the vapor pressure of water at room temperature (25 ° c) is 23.8 mm hg, 0.0313 atm, or 23.8 torr, or 3.17 kpa. It is the pressure exerted by the saturated vapour in contact with the surface. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). At its freezing point (0 ° c), the vapor pressure of. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. Equation 2 ln p w, s = c 8 /t + c 9 + c 10 t + c 11 t 2 + c 12 t 3 + c 13 ln t. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa. Web the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling temperature for water. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). Equation 2 ln p w, s = c 8 /t + c 9 + c 10 t + c 11 t 2 + c 12 t 3 + c 13. The output temperature is given as °c, °f, k and °r. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Web vapor pressure of water (mmhg) at selected temperatures. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Vapor pressure of water is shared under a not. Web vapor pressure of water (mmhg). Web water vapour pressure table at different temperatures. Web the vapor pressure of water at room temperature (25 ° c) is 23.8 mm hg, 0.0313 atm, or 23.8 torr, or 3.17 kpa. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Pressure (degrees c) (mmhg) (degrees c) (mmhg) Web vapor pressure of water (mmhg) source of data: Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. Pressure (degrees c) (mmhg) (degrees c) (mmhg) Web the calculator below can be used to calculate the water boiling point. Web for example, as water boils at sea level, its vapor pressure is 1 atmosphere because the external pressure is also 1 atmosphere. Web vapor pressure of water from 0 °c to 100 °c. The pressures are stated in. It is the pressure exerted by the saturated vapour in contact with the surface. Web vapor pressure of water (mmhg) at. Web water boiling temperature vs pressure in vacuum table chart. A plot of the vapor pressure of water versus the water's temperature. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa. Web what is. Web the vapor pressure of water at room temperature (25 ° c) is 23.8 mm hg, 0.0313 atm, or 23.8 torr, or 3.17 kpa. Web water vapour pressure table at different temperatures. Pressure (degrees c) (mmhg) (degrees c) (mmhg) It is the pressure exerted by the saturated vapour in contact with the surface. Web for example, air at sea level,. Atomic parameters (ie, ea, d,.) thermodynamic data. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. Web vapor pressure of water from 0 °c to 100 °c. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the. Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Web for example, as water boils at sea level, its vapor pressure is 1 atmosphere because the external. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). The output temperature is given as °c, °f, k and °r. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of. Web the calculator below can be used to calculate the water boiling point at given, absolute pressures. The line on the graph shows the boiling temperature for water. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa. At its freezing point (0 ° c), the vapor pressure of. Web what is the vapor pressure of a solution made by dissolving 100 grams of glucose (c 6 h 12 o 6) in 500 grams of water? The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the. Atomic parameters (ie, ea, d,.) thermodynamic data. Web the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. A plot of the vapor pressure of water versus the water's temperature. Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Web vapor pressure of h 2 o at various temperatures (celsius) note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into.Vapor Pressure Chart For Water
Conservation physics Fundamental microclimate concepts
Vapor Pressure Chart For Water
Vapor Pressure Chart For Water
Vapor Pressure Chart For Water
Table Of Vapor Pressure Of Water A Guide To Understanding Its
Vapour Pressure Of Water Chart
[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A
Water Vapour Pressure Chart Bar
Water Vapor Pressure Chart
Web For Example, As Water Boils At Sea Level, Its Vapor Pressure Is 1 Atmosphere Because The External Pressure Is Also 1 Atmosphere.
Web Water Vapour Pressure Table At Different Temperatures.
Vapor Pressure Of Water Is Shared Under A Not.
Crc Handbook Of Chemistry And Physics, 84Th Edition (2004).
Related Post:





![[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A](https://d3i71xaburhd42.cloudfront.net/471011a1b864be3f78720f4d8ba4f21385acd117/5-Table4-1.png)

